NCDC confirms 663 news COVID-19 cases
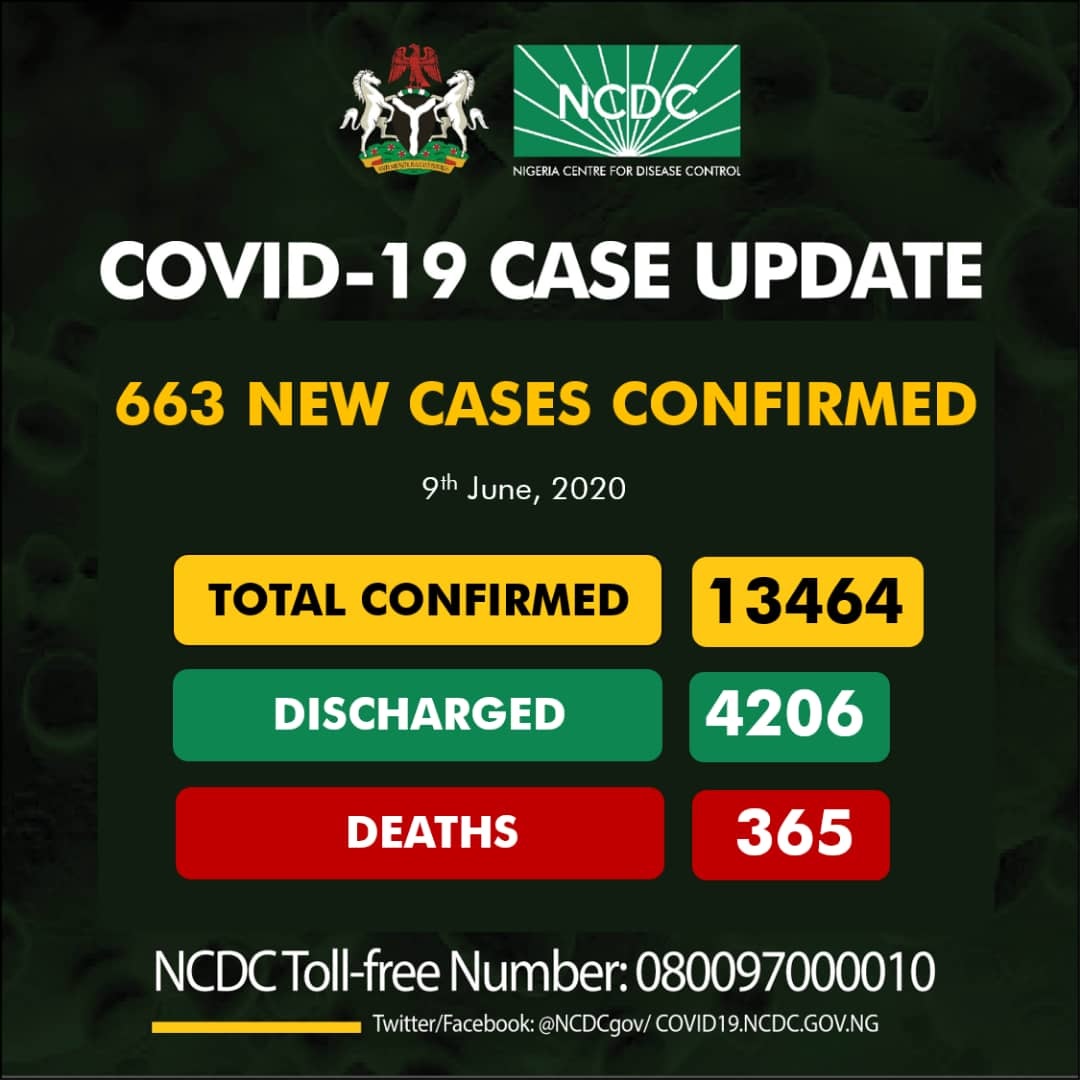
The Nigeria Centre for Disease Control (NCDC), on Tuesday, confirmed 663 new cases of Coronavirus in the country, the highest daily figure of confirmed COVID-19 cases infections so far.
With the latest update, the total tally of infected people in the country rose to 13, 464 from 12, 801 reported on Monday evening.

Previously, the highest daily number recorded was 553 on May 31.
Four deaths were recorded from the virus on Tuesday bringing the total number of confirmed deaths from the virus to 365.
The health agency in a tweet on Tuesday night said the new cases were reported in 26 states. These are Lagos, Ogun, Bauchi, Ebonyi, Edo, Rivers, Federal Capital Territory, Jigawa, Delta, Anambra, Gombe, Kano, Imo, Abia, Borno, Oyo, Plateau, Kebbi, Kaduna, Ondo, Niger, Katsina, Osun, Ekiti, Kwara and Nasarawa States.
All the reporting states already had at least a case of the virus.
As of the time of reporting, 35 states and the FCT have recorded at least a case of the disease. Only one state, Cross River, is yet to report any case of the virus.
In the past weeks, the numbers of infections and deaths in the country have increased. The number of recovered and discharged patients from the virus is also increasing daily.
“Till date, 13, 464 cases have been confirmed, 4206 cases have been discharged and 365 deaths have been recorded in 35 states and the Federal Capital Territory,” it said.
Customs handovers seized Cannabis sativa to NDLEA in Kwara
The 663 new cases are reported from 26 states- Lagos – 170, Ogun – 108, Bauchi – 69, Ebonyi – 49, Edo – 33, Rivers – 30, FCT – 26, Jigawa – 26, Delta – 20, Anambra – 17, Gombe – 16, Kano – 16, Imo – 15, Abia – 14, Borno – 11, Oyo – 11, Plateau – 8, Kebbi – 6, Kaduna – 6, Ondo – 4, Niger – 2, Katsina – 2, Osun – 1, Ekiti – 1, Kwara – 1 and Nasarawa – 1.
Meanwhile, the Health agency says it has activated three additional laboratories, bringing the total number that can carry out PCR testing for Coronavirus (COVID-19) pandemic in Nigeria to 33.
Dr Chikwe Ihekweazu, the Director-General of NCDC, told the News Agency of Nigeria (NAN) on Tuesday in Abuja that the health agency had stayed on track in its goal to rapidly scale up laboratory testing for COVID-19 in Nigeria.
Ihekweazu mentioned the three new laboratories as: Biorepository and Clinical Virology Laboratory UCH, Ibadan, Oyo; Molecular Diagnostics Laboratory, Infectious Disease Unit, General Hospital, Ituk Mkpang, Akwa-Ibom and Jigawa State Molecular Laboratory, Dutse.
He said that NCDC was currently using the Polymerase Chain Reaction (PCR) method to test for the COVID-19 virus, in the absence of validated alternative methods.
“At the moment, the COVID-19 tests that we report daily are coming from the PCR, they detect the genetic information of the virus and the RNA. That’s only possible if the virus is there and someone is actively infected.
“By detecting viral RNA, the tests can tell whether or not someone has the virus very early on,” he explained.
The director-general said that PCR remained the most accurate method to determine who was infected.
Ihekweazu noted that by scaling up on laboratory testing, the NCDC and other relevant government institutions would get a better understanding of the level of spread of the virus.
”By the end of June, our goal is to include at least 10 more laboratories with current Gene-Xpert capacity in the network for COVID-19 testing,” Ihekweazu said.
The director-general noted that PCR tests could be very labour intensive, with several stages at which errors might occur between sampling and analysis.
”This is why the agency has focussed on strengthening quality assurance in its network of laboratories.
“Countries have adopted varying strategies for COVID-19 diagnosis, and for us in Nigeria, it is important that we get it right.
“Nigeria has its national testing strategy for COVID-19 with detailed information on how we will ramp-up testing for various phases of transmission by leveraging existing capacity and technology.
“We are thinking ahead and also have plans in place to meet the demands for testing at various points of the response,” he said.








